Electrolysis Advanced Experiment
Background
They’ve been seen everywhere together—at ice skating rinks, in hot tubs, standing on city sidewalks, or running through storm drains—hydrogen and oxygen atoms, bonded together to form molecules of water. Sure, they’d disappear into thin air for a while, even go underground. But they always returned—together. It’s shocking to hear what’s come between them: a chemical reaction called electrolysis.
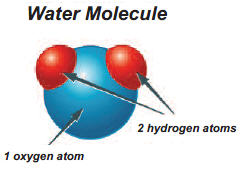
Electrolysis is a decomposition reaction. A decomposition reaction is a type of chemical reaction in which a compound breaks down into its basic elements. Water is a compound made from the elements hydrogen and oxygen.
During electrolysis, electricity causes the hydrogen and oxygen atoms in water to dissociate, or split apart. Try the following experiment and watch these former partners go their separate ways.
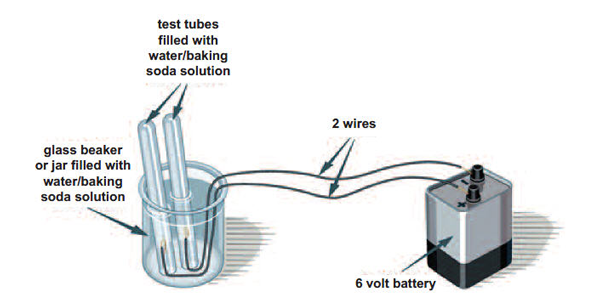
Materials
- 1 glass beaker or jar
- 2 test tubes or clear vials
- 6-volt battery
- two 8-inch pieces of coated wire (18-22 gauge) with ends stripped
- 2 cups tap water
- 2 tbsp. baking soda
- 1 measuring cup
- 1 measuring spoon
- wire cutters
Steps
- Mix 2 tablespoons of baking soda in the 2 cups of water. Stir until the baking soda has dissolved. Then pour about 1¼ cups of the solution into a beaker or jar.
- Set up the experiment as shown, leaving one of the battery wires unconnected at this point. You will need to fill each test tube to the top with the leftover solution, then hold your finger over the open end as you immerse it upside-down in the beaker of solution. Position one tube over each wire tip.
- Connect the loose battery wire and observe what happens at the tip of each wire in the solution. Let the reaction continue for at least five minutes.
Questions
- The chemical formula for a molecule of water is H2O: two atoms of hydrogen bonded to one atom of oxygen. Look at the test tubes.
Which test tube contains more gas? _______________________________Which test tube do you think contains hydrogen gas? _______________________________Which contains oxygen gas? _______________________________
- The baking soda was added to the water to speed up the electrolysis reaction. What would be another way to speed it up?
- What do you think would happen if you repeated the experiment with a hydrogen chloride solution instead of water? Why would this be dangerous?

